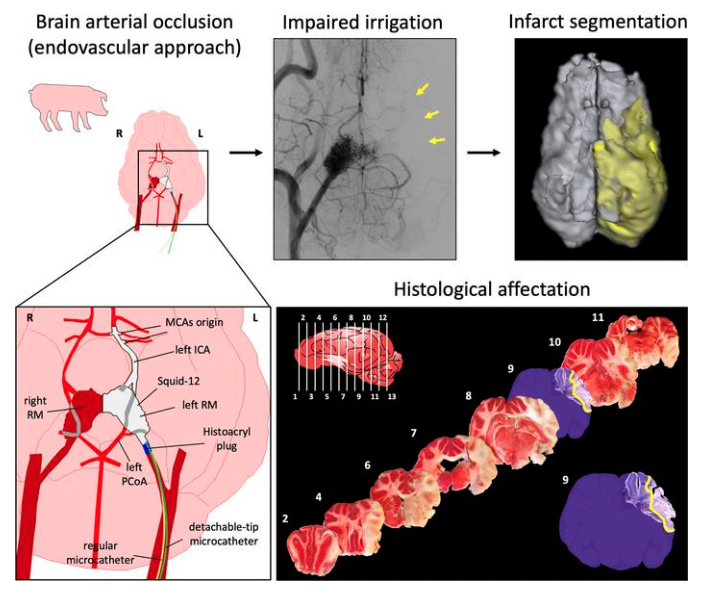
Researchers of the Cellular and Molecular Neurobiology (CMN) Research Group at the Germans Trias i Pujol Institute (IGTP) have developed and established a novel, reproducible and minimally invasive stroke model in the pig through an endovascular approach. The work has been conducted at the Centre for Comparative Medicine and Bioimage (CMCiB), a centre devoted to translational medicine at the very core of the Can Ruti Campus in Badalona together with the IGTP and the Germans Trias i Pujol University Hospital.
This recent advance fulfils the requirements needed to promote an efficient translation of innovations and new treatments from the bench to the bedside in this field. The study is published in the April 2023 issue of the Journal of Clinical Investigation Insight as a remarkable Technical Advance that mimics the occlusion at the same cerebral artery site reported in the most widely used experimental stroke model in mice and rats, but using the pig as the experimental subject for its brain is far more similar to that of humans than rodent’s. The need for animal models of stroke to search for and to find new treatments in species with more human-like brain characteristics as compared to rodents, but avoiding the use of non-human primates to fulfil the EU regulations and to fit ethical concerns, is a long-pursued demand of stroke. In this regard, this novel stroke model in pig will allow to better model and keep on studying the mechanisms operating in stroke that are also present in stroke patients, and to find stroke therapies useful to alleviate patients. The new technique described produces an effective occlusion of the cerebral arteries of interest, induces early focal brain damage at the desired cerebral regions 90 min post-occlusion that later evolves to a large cerebral infarction, and produces neither mortality during the intervention nor surgical-derived discomfort. This novel ischemic stroke model in pigs shows translational features strikingly common to human stroke, including damage to the white matter axonal tracts that transmit information interconnecting specific functional brain areas. In this model, the researchers used longitudinal multimodal cerebral MR imaging similar to the one currently used in the clinical set up to assess the evolution of brain damage and cerebral blood supply.
“The reproducibility of the damage in specific brain areas in the model is important to any study that aims to determine the true neuroprotective effect of new molecules to be tested in brains similar to those of humans“, says Dr. Teresa Gasull, who has led the study. In words of Dr. Carlos Castaño and Marc Melià-Sorolla, co-first authors of the study, “We expect this new model will foster the development of new therapeutic compounds and devices to treat patients in the years to come”.
Share:
Linkedin
Twitter