- Contact us
- Request a quote
Services
Specipig is a Contract Research Organization (CRO) focused on the swine model.
We offer customized services specialized in the pig model, with preclinical support for research centres, hospitals, biomedical, pharmaceutical, biotechnological and medical device companies in all phases of their projects.

Specipig is a Contract Research Organization (CRO) focused on the swine model. Almost 10 years’ experience with more than 150 projects and customers from more than 14 countries endorse our expertise and capacities.

Our team is highly qualified in swine management, breeding, health and welfare.
We are experts on the swine model, with more than 2500 m2 housing facilities, with a breeding centre and more than 300 animal integrated in the same facilities. Enabling our team to acquire expertise and be highly qualified in swine management, breeding, health and welfare.

Specipig has developed a unique breed of miniature pigs. Facilitating the control of the supply chain and gaining in knowhow of the pig model.

We are fully GLP certified and entirely compliant with the guidelines issued by ICH, EMA, ISO 10993, FDA and other relevant health authorities.
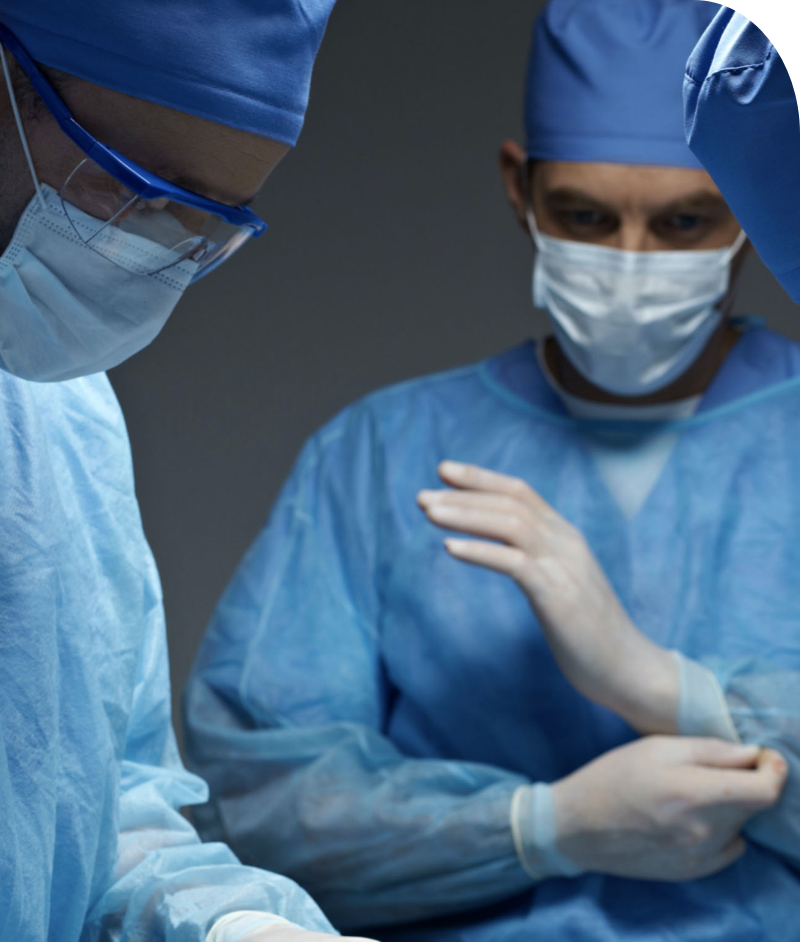
A wide team of collaborators on different surgical services and expertise empower Specipig with high competences otherwise difficult to achieve.

Specipig has a high customization capacity, given its small structure that provides agility and quick response to the demands.
Biopharma
Specipig has experience in running drug development in-vivo pig studies to overcome the regulatory requirements. Such as proof of concept, efficacy, pharmacokinetic, multi dose tolerance studies, toxicology studies and skin tolerance trials. All under GLP certification.
Our aim is to accompany our partners all along the process, from the very beginning of the preclinical phase, overcoming the regulatory requirements.
Proof of concept
Efficacy studies
PK / TK
Toxicology
Medical device
In-vivo studies to test medical devices, from a prototype level to a regulatory phase, are possible at Specipig. We support medical device companies to establish the best approach to run proof of concept in-vivo studies of new devices or tissues.
We provide the best experts in each area (human or veterinary medicine surgeons) and open our doors to surgeons provided by the sponsors to support with the implantation or placement procedure.
We overcome the necessary steps to support a project during the regulatory submission of the in-vivo pig preclinical studies. Running them under GLP certification, when necessary.
Our aim is to accompany our partners all along the process, from the very beginning of the prototype phase, run verification and usability tests, and to overcome the regulatory requirements, in fully equipped operation rooms and with the support of an in-vivo large animal pig model.